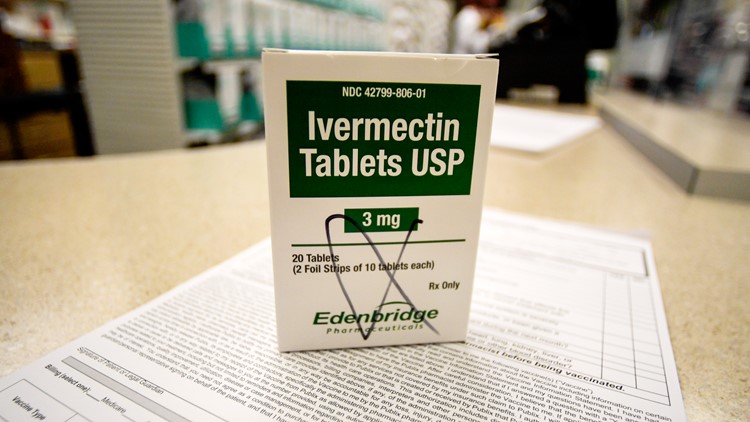
TENNESSEE, USA — Vanderbilt University Medical Center is looking for people to participate in a study to see if ivermectin and other drugs can treat COVID-19.
Ivermectin is a drug primarily used to treat parasitic infections. The drug was originally discovered in the 1970s and later formulated as a veterinary drug to treat parasitic infections in horses and livestock such as heartworm, which it is still widely used for this purpose in the U.S. in the form of ivermectin paste.
It was later found in the 1980s to have practical uses in much lower doses for humans as a cost-effective treatment against parasites to combat malaria and other diseases plaguing less developed parts of the world. For people in the U.S., it is sometimes prescribed as a pill or topical treatment for head lice and rosacea.
In 2021, ivermectin gained notoriety after several poison control centers across the country had reported seeing a sudden rash of people who had overdosed on ivermectin horse paste, which can be bought without a prescription. The Food and Drug Administration said some people ended up in the hospital after they tried to self-medicate with large doses of ivermectin horse paste based on misinformation they saw on social media about the drug and how to obtain it.
Even though ivermectin is intended as an antiparasitic, clinical studies are still being conducted on the drug to see if it also has practical antiviral applications as a cost-effective treatment for COVID-19, but so far researchers said it is still too early to tell if it is safe or effective as a treatment against the virus.
Researchers at Vanderbilt University studying the drug's application as a COVID-19 treatment said the misinformation people have shared online misrepresenting studies still being conducted has been dangerous.
“There is so much misinformation about ivermectin that’s out there,” said Dr. Parul Goyal, the principal investigator in the study at Vanderbilt.
Carolyn Coleman was diagnosed with COVID in early November, one day before she was scheduled to get her booster shot.
“I’m double vaccinated,” said Coleman. I want to do anything I can to help the science advance and help people, you know, stay well.”
While Coleman has COVID-19, she decided to take part in the remote Activ-6 Study and help COVID-19 in the long term. In the study, several hundred people across the country receive one of three medications:
- Ivermectin
- Fluvoamine (an anti-depressant)
- Fluticasone (asthma treatment)
- Participants may also receive a placebo
Coleman said she received fluticasone.
“I didn’t pick that one. It was picked for me,” said Coleman.
Goyal said most people who take part in the study are interested in ivermectin.
“The dose of the ivermectin that we are using in the study is a relatively lower dose and it’s a weight-based prescription,” said Goyal.
Goyal said ivermectin comes in a pill meant for humans. It’s only 7 milligrams compared to the massive doses given to heavier animals like horses.
“Ivermectin that was available for livestock is a lot more dangerous,” said Goval. "The side effects are much more as the dose is highly concentrated.”
“I don’t feel any worse, but I was also on the mend anyway,” said Coleman.
She mentioned she didn’t notice a difference in her symptoms with fluticasone, but she wouldn’t say no to ivermectin.
“I don’t know anything about ivermectin. I would have taken it because it was the agreement I made with the study,” said Coleman.
To take part in the study, you must be at least 30 years old and have two symptoms related to COVID-19. To sign up, visit the website or call 615-343-8010.