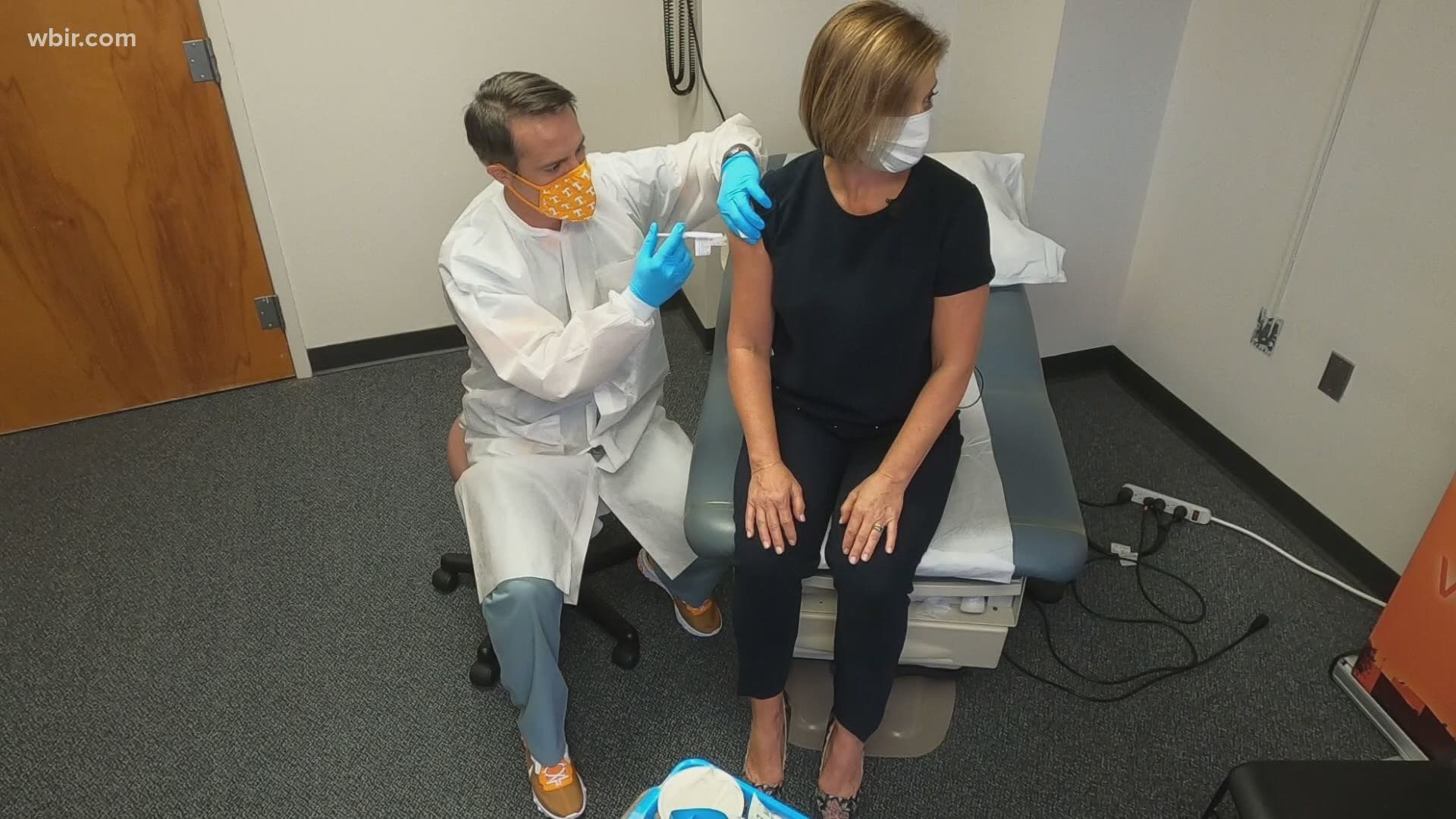
KNOXVILLE, Tenn. — When WBIR’s Robin Wilhoit decided to volunteer for an experimental COVID-19 vaccine trial through Volunteer Research Group at UT Medical Center, she wanted to document the process to provide a first-hand account.
She is one of more than 40,000 people across the nation participating in the trial run by the pharmaceutical giant, Pfizer. This human clinical trial for the COVID-19 vaccine requires two injections and takes place over the course of 24 months.
The first visit will determine if you qualify for the trial and then the first of two injections.
For more on Robin's vaccine trial experience: click here
You will then return to the medical center for a second dose. Much like the first visit, doctors test you for coronavirus to make sure you have not been infected since the first dose since there is a 50/50 chance of either receiving the real vaccine or a placebo.
The only people who know whether the injection is the real vaccine or a placebo are the researchers at Pfizer and the pharmacist who administered the first shot.
If the coronavirus test is negative, a pharmacist administers the second vaccine.
This vaccine does not contain the coronavirus, rather it contains mRNA that can teach your body how to fight off the virus.
Like the first dose, you record your temperature and any side effects for the next seven days in a diary through an app.
It is important to note that you may not react the same way between the two doses.
Robin experienced only soreness at the injection site for the first vaccine but woke up in the middle of the night with a 100-degree fever that lasted for roughly a day after the second vaccine.
"[Volunteer Research Group's Dr. Bill Smith] said these side effects could happen. They do happen, but these are things that are very manageable. It's not something that's got me down and out," Robin said in a video diary from her couch the day after her second dose.
She said she was fine after 24 hours.
"Absolutely, it's worth it. All little downtime, a little discomfort is well worth it if they get the information they need from all the people who are getting the doses in order to determine whether this vaccine is effective," Robin said in a video diary two days after her second shot.
The trial does not stop there though. Fast forward 3-4 weeks and it’s back to UT Medical Center for a blood draw.
The blood will be processed and analyzed for the antibody levels, which will tell researchers how your body responded to the vaccination.
If you got the real vaccine instead of the placebo, it’s likely you have developed antibodies that will protect you from COVID-19.
After this initial blood draw, you return every few months for additional tests to make sure the vaccine is still working.
"So far we've seen, in the data that's available, we've seen antibody responses that are even higher than what is seen with having the infection itself. So that bodes well for the fact that the vaccines are likely to work," said Dr. Bill Smith with Volunteer Research Group.
Pfizer said it expects to have early results by the end of October. If that is the case and the vaccine is vetted and approved, people can start getting vaccinated.