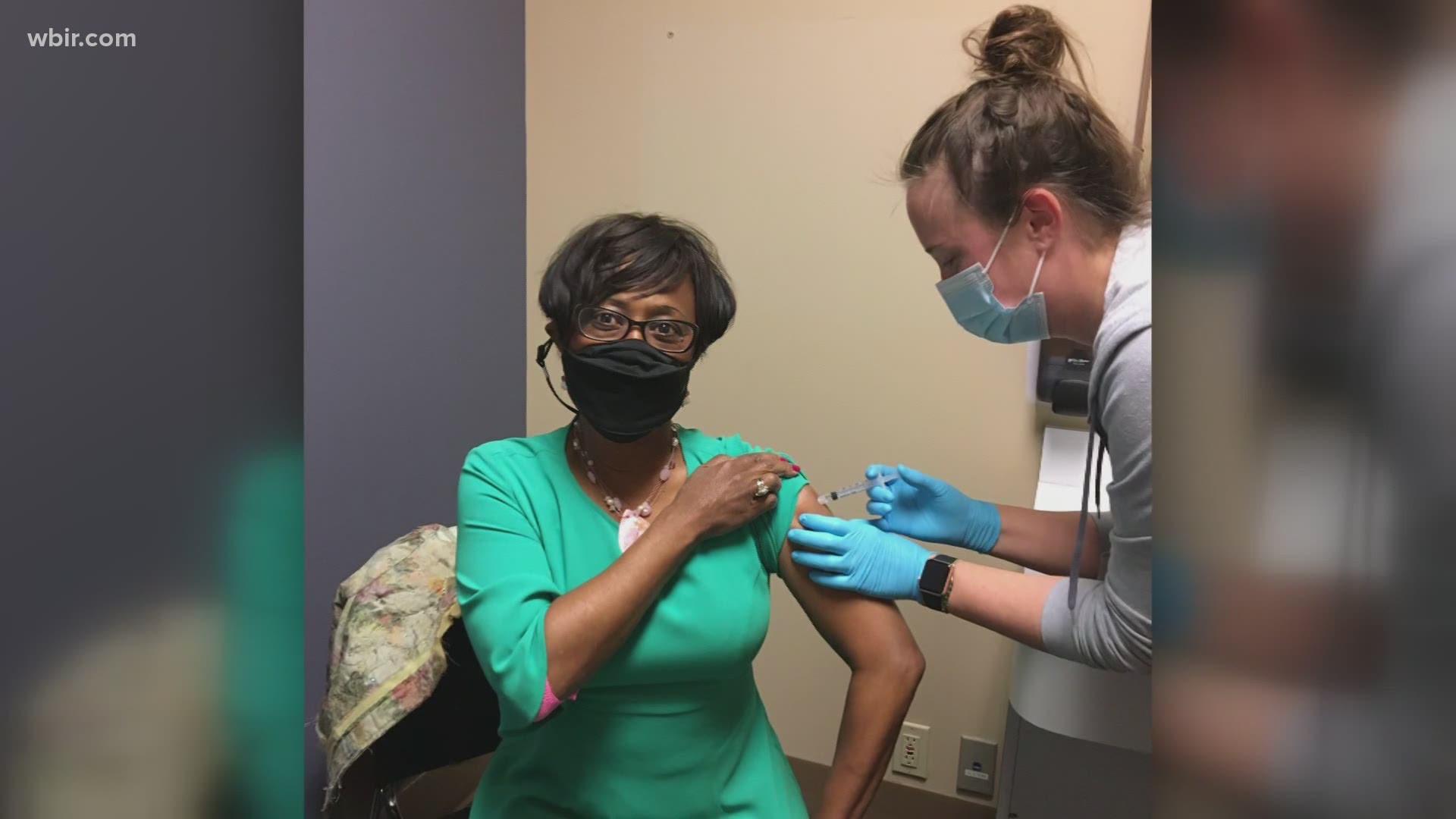
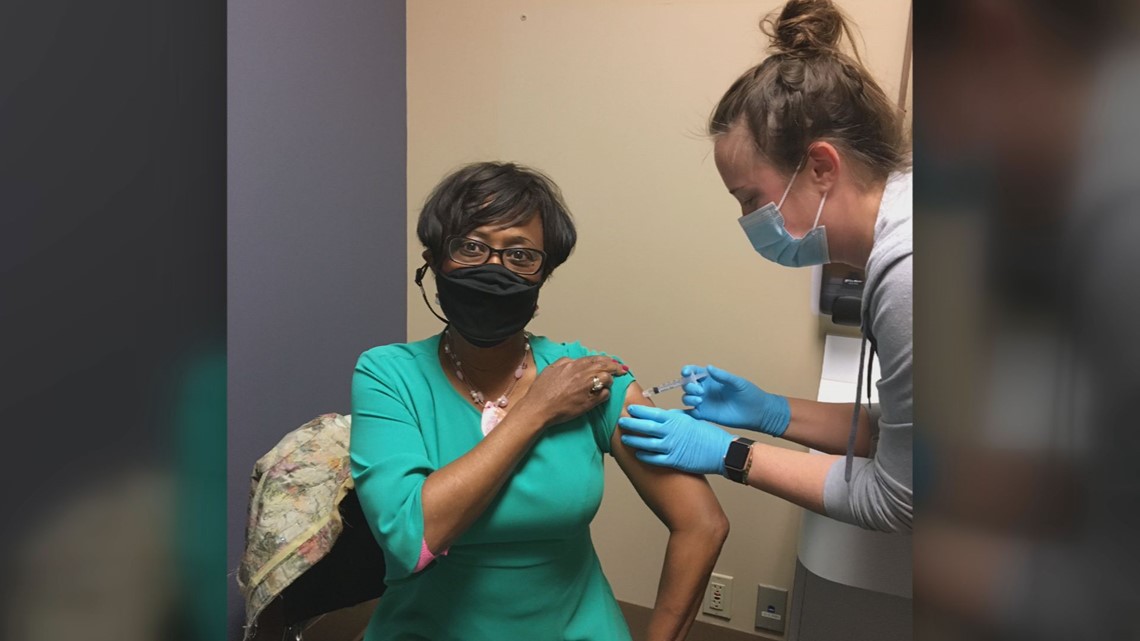
KNOXVILLE, Tenn. — For Cynthia Finch, signing up for a vaccine trial just felt like the right thing to do. She's a clinician, community advocate and person of color.
"I want to set an example that I took my shot," Finch said. "It's important for people of color to be a part of a process to make sure things get right."
Plus, she knows some people are hesitant to get the COVID-19 vaccine — especially African-Americans.
"I know that statistics have shown that African Americans and people of color have been hesitant to take the vaccine for many different reasons," Finch said. "Many of them very, very legitimate because of the history of challenges with being involved in clinical trials processes"
Dr. William Smith is overseeing the AstraZeneca trial participants at AMR Knoxville. He said they are looking for roughly 2,500 more minority participants across the United States.

"The safety profile for this is well established. It's more traditional technology and there are now over 50,000 people worldwide that have received it," Dr. Smith said. "The missing portion are minorities."
Once they have a more representative sample, the AstraZeneca study can be closed and presented to the Food and Drug Administration.
"Every additional vaccine that we get just enables us to vaccinate more people faster and to reach the point where we can start to get back to normal," Dr. Smith said. "This is really critical to help finish the study."
Finch does not know whether she received the vaccine or the placebo, but she's working to get her five brothers to participate, too.
She's also trying to talk to 25 people a day about her experience and why she trusts the vaccine.
"When you're informed, when you're knowledgeable and when you have access, then you will say yes," Finch said. "How do we move people from no to maybe to yes? I want us to be able to be trustful."