Pfizer announced its COVID-19 vaccine had had nearly 95% percent effectiveness in a clinical trial check-in. On Nov. 18, the Pfizer-BioNTech vaccine study phase 3 trials had concluded, leaving it next in the hands of the FDA to issue an Emergency Use Authorization.
It is one of several COVID-19 vaccines being developed. The Pfizer vaccine is an mRNA vaccine. Unlike the shots we're used to, it doesn't contain the coronavirus at all. Instead, it targets cells inside the body. In essence, it speaks to proteins and teaches them to produce antibodies on their own.
As vaccines are developed, viewers asked whether there would be side effects from them.
Usually, the Food and Drug Administration releases Vaccine Information Statements for vaccines. Several are available for major vaccines including the DTaP, Hepatitis A, HPV and Polio vaccines. The statements list several facts about the vaccines, including risks of different reactions.
Doctors are required under federal law to give the VIS to any patient before administering a vaccine. People who receive the COVID-19 vaccine can expect to get the sheet. A group of doctors also asked the CDC to warn people about possible side effects from the vaccine.
They said that they are worried people may be surprised by the possible side effects from the first shot of the vaccine. If they are surprised, they may get the necessary second shot later, which is required from the vaccine to work.
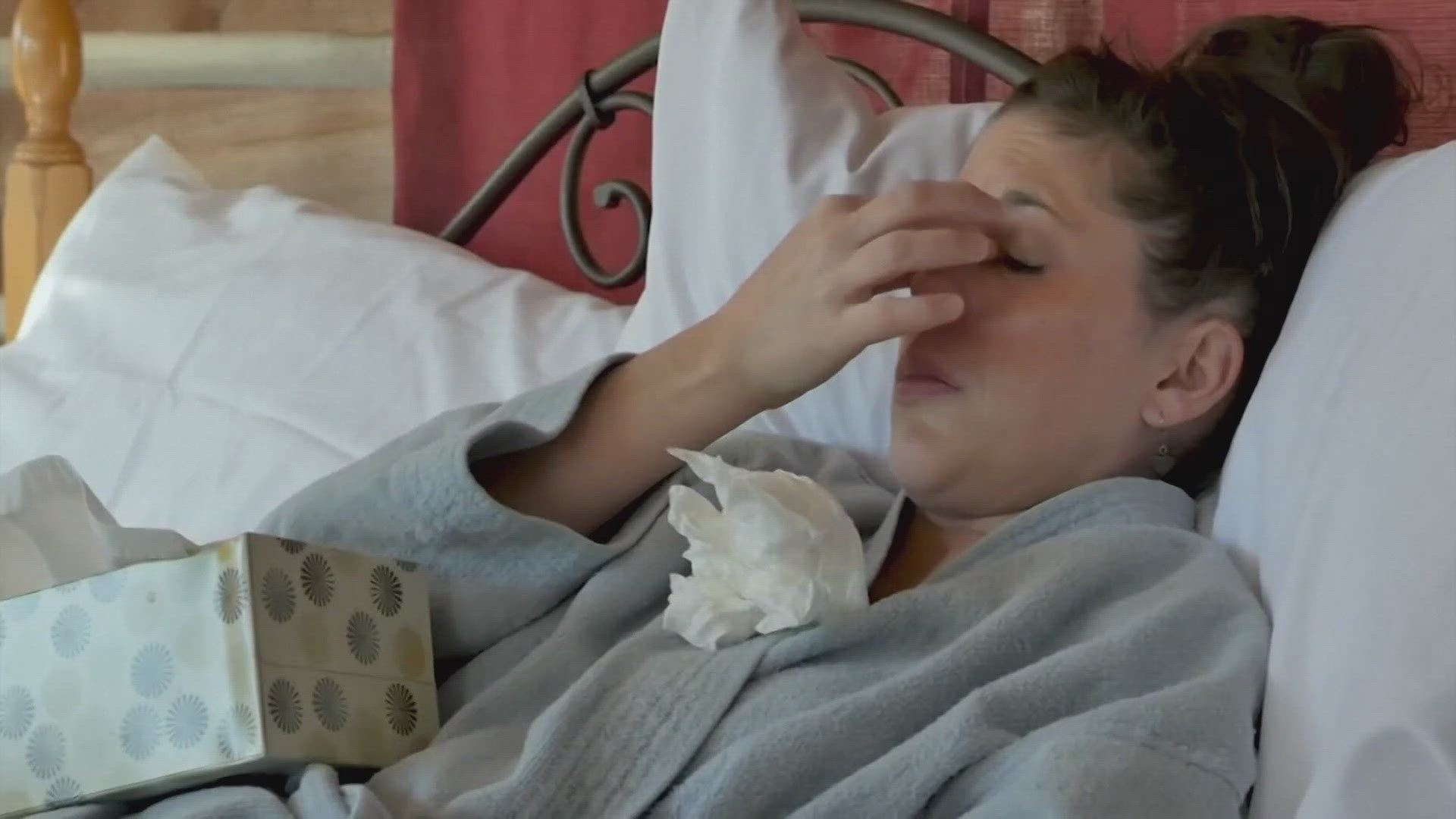
WBIR's own Robin Wilhoit was a participant in trials for the Pfizer COVID-19 vaccine. She said that after the second shot, she had a low-grade fever, chills and felt aches.
However, she also said that the symptoms only lasted around 24 hours and once they were gone, she felt back to normal.
Tennessee officials said that they hope to get the first of its COVID-19 vaccine doses in mid-December.